Search keywords:
product name, product type, model number,
test method, manufacturer, technique, application
Inhaler Test Equipment
BACKStandards:
Applications:
Product Information:
Introduction
The inhaler test device is accordance with the requirements of the United States Pharmacopeia USP and the European Pharmacopoeia (Ph. Eur.).
The wide range of products and services is designed to meet all the routine R & D and QC testing requirements of inhalers, including:
1. Metered-dose inhalers (MDI or pMDIs);The wide range of products and services is designed to meet all the routine R & D and QC testing requirements of inhalers, including:
2. Dry powder inhaler (DPIs);
3. Sprayer (water inhaler);
4. Nasal sprays and aerosols.
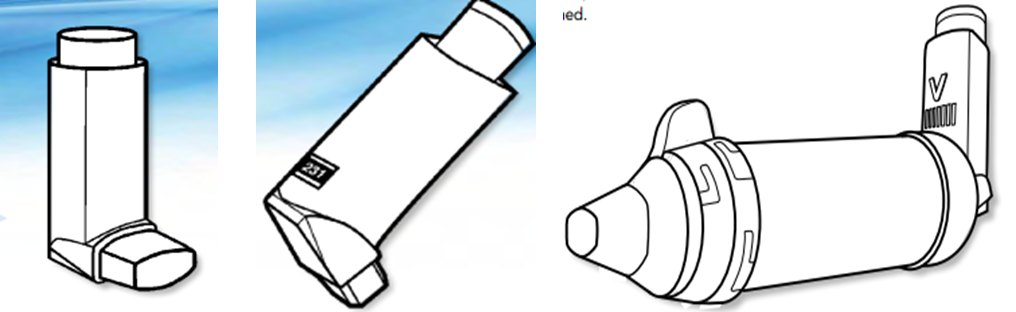
Metered-dose inhalers With metered-dose and meter counter With valve room MDI
(MDI) (MDI)
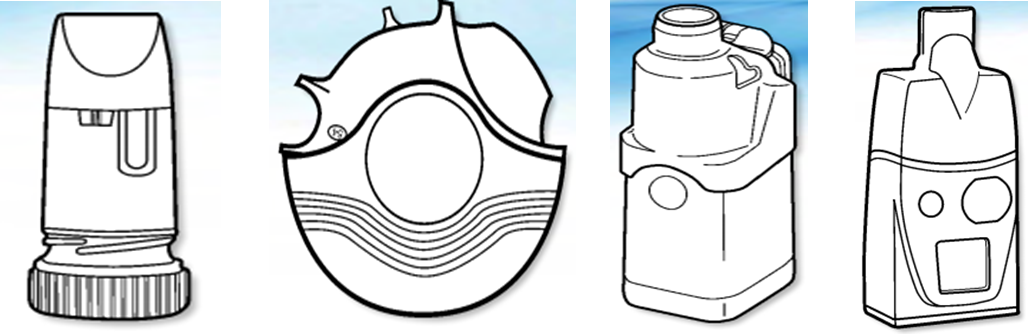
Dry powder inhaler Dry powder inhaler (Predicted) Ultrasonic sprayer Sieve pore Sprayer
(Equipment metering)
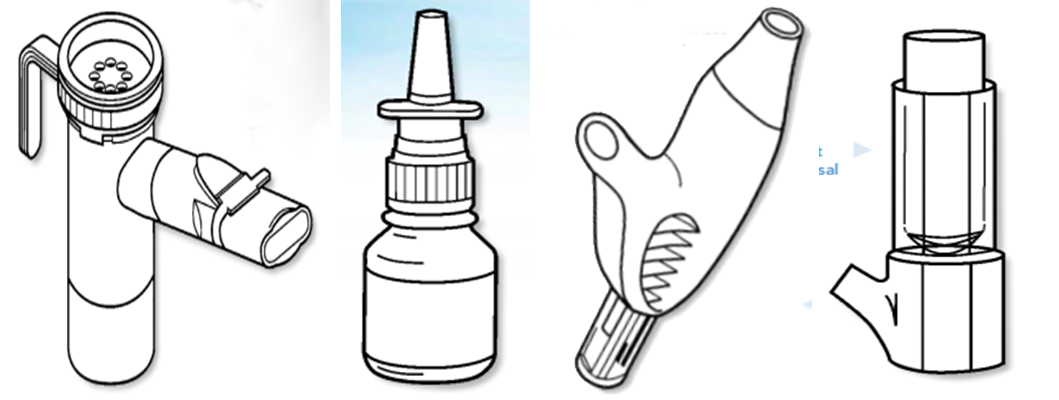
Jet type atomizer Nasal spray pump dose Powder nasal spray device Push type nasal aerosol
Product Function
· Dose uniformity
· Uniformity for the whole drug ingredient dose
· Cascade impact aerodynamic particle classification
· Critical (sound) flow control and measurement
· System suitability test
· data analysis
· Force saving device
· The IQ / OQ document of the calibration, maintenance and impact phase measurement services.